Executive Summary (TL;DR)
- Hop latent viroid (HLVd) is a small, circular RNA viroid that moves primarily by mechanical transmission (sap on tools/hands). It reduces vigor, yield, and quality (terpenes/cannabinoids) and can silently spread through mother rooms and clone pipelines.
- Prevention beats cure: implement facility zoning, sanitation that actually inactivates viroids, clean-stock sourcing, and mother indexing on a schedule. RT-qPCR is the lab standard for detection; bleach/heat are validated tool-sanitation methods.
- Real estate matters: design flow for clean → dirty with space for quarantine, sampling, and tool bake/chemical stations. If time-to-recovery matters, acquire a clean operating platform and retire infected stock. → Find cultivation/production businesses for sale
- Use the decision matrix and SOPs below to harden biosecurity and keep your mother/clone supply running while you test, cull, and re-stock.
Table of Contents
- What HLVd is (and why it behaves differently from a virus)
- How HLVd spreads in real facilities
- Business impact: yield, potency, and brand risk
- Detection: sampling strategy, assay options, and cadence
- Prevention architecture: people, tools, rooms, and workflow
- Mitigation: triage, sanitation that works, and clean-stock rebuilds
- Decision matrix and SOPs you can deploy this week
- Facility & siting considerations (zoning, buffers, and build-outs)
- Next steps and curated inventory for faster recovery
What HLVd is (and why it behaves differently from a virus)
HLVd (Hop latent viroid) is not a virus; it’s a viroid—a minimal, non-coding circular RNA that hijacks host machinery. Because viroids lack protein coats, their stability and inactivation profile differ from many viruses; that’s why some common disinfectants and even autoclaving do not reliably inactivate HLVd in operational settings. The result: a pathogen that can ride blades and gloves from plant to plant with little warning, causing the familiar “dudding” phenotype—reduced vigor, shorter internodes, poor trichome density, and yield/potency losses. Authoritative surveys and reviews have documented high prevalence and significant crop and economic impacts in legal cannabis production. PMC+1Terra Vera
Primary keyword note: This guide uses “HLVd” and “hop latent viroid” throughout to help cultivators searching for “HLVd prevention,” “HLVd detection,” or “HLVd sanitation” find a single, comprehensive playbook.
How HLVd spreads in real facilities
Dominant route: Mechanical transmission—infected sap entering micro-wounds on clean plants—during topping, pruning, grafting, and cloning. Poor tool discipline and glove reuse amplify spread, especially in mother and clone rooms. TUMI GenomicsPMC
Other routes supported by evidence:
- Roots and shared nutrient solution: side-by-side rooting or recirculated fertigation can move HLVd between plants. MDPI
- Plant-to-plant contact via workers and benches: contaminated hands, aprons, or surfaces bridge sap between cut sites.
- Seeds/pollen/insects: evidence is mixed and cultivar-dependent; assume possible low-rate seed transmission and mechanical assistance by insects when sap is exposed, but build your program around tool/human/solution control, which are proven dominant. PMC
Business impact: yield, potency, and brand risk
HLVd rarely kills plants; it quietly destroys unit economics:
- Yield: significant reductions (room-scale reports of double-digit losses) tied to reduced vigor and flower formation.
- Potency & terpenes: lower secondary metabolite expression leads to weaker COAs and downgraded grades.
- Mother/clone pipeline: infection in stock plants ensures every batch of cuts carries risk, compounding losses every cycle.
- Cash conversion: testing, culling, and re-propagating extend production cycles and tie up rooms.
Peer-reviewed reviews and industry surveys have quantified substantial, sector-wide losses and high facility prevalence, underscoring HLVd as a top-tier operational risk that must be budgeted in every business plan. PMC+1
Detection: sampling strategy, assay options, and cadence
What to test
- Mother stock (highest priority), new inbound clones, and any symptomatic benches.
- Tissue choice: petioles from symptomatic leaves, meristematic tissue, or crown/roots (HLVd can titer higher in root/crown tissues). When symptoms are patchy, take composite samples (multiple leaves/petioles) per plant to improve sensitivity.
Assay options
- RT-qPCR (reverse-transcription quantitative PCR) is the current gold standard for HLVd detection—sensitive and specific when validated with proper internal controls. Commercial kits and lab SOPs are widely available. 3402974.fs1.hubspotusercontent-na1.netbioreba.chpinnacle-analytics.com
- RT-LAMP (loop-mediated isothermal amplification) offers faster, field-friendly screening with somewhat lower specificity/quantitation than qPCR, useful for triage before confirmatory RT-qPCR. MyFloraDNA
Quality control for assays: Ask your lab about their internal amplification control (to catch PCR inhibition), matrix validation for your tissue type, and limit of detection (LOD). Industry guidance recommends internal cannabis mRNA controls in RT-PCR workflows to ensure sample quality. Cannabis Business Times
Cadence & thresholds
- Inbound plant material: Quarantine and test every lot of clones or mothers before entry to production.
- Mother rooms: Index every 2–4 weeks depending on cut frequency; remove or isolate positives immediately.
- Rooms with symptoms: Test representative plants at bench ends and near workflow chokepoints (trim stations, doorways).
- Release criteria: Define “clean” as two consecutive negatives (7–14 days apart) before re-entry of quarantined stock.
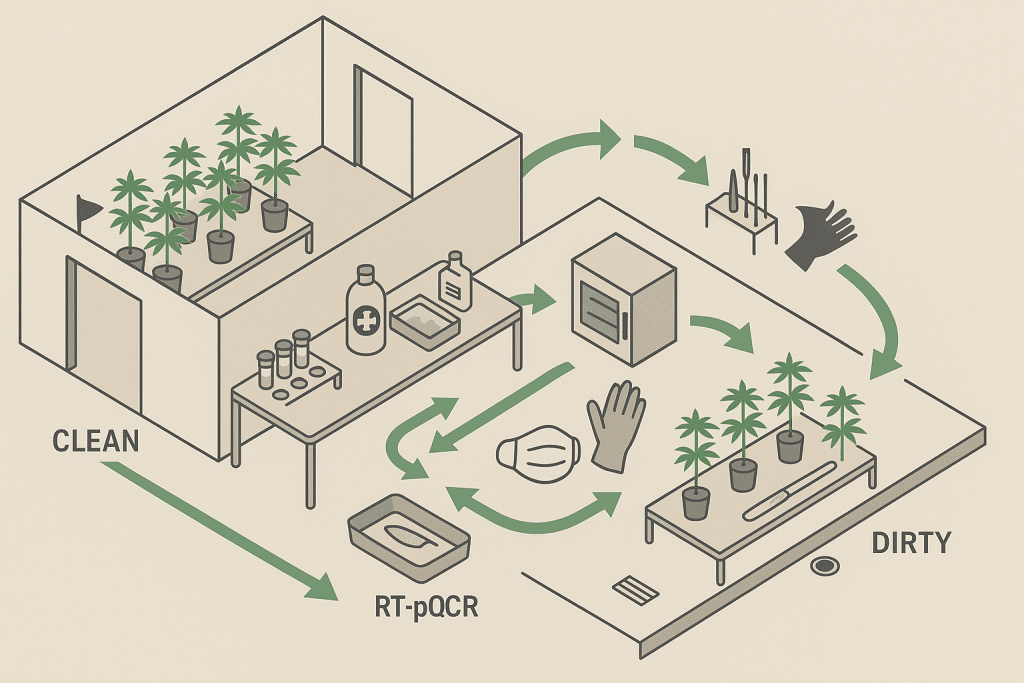
People and flow
- Zoning: Work clean → dirty (tissue culture/quarantine → mothers → propagation → veg → flower → trim/waste). Never backtrack upstream without gowning changes.
- Gowning: Gloves and aprons per room; change gloves between benches and whenever blades are sanitized.
- Scheduling: Start the day with the cleanest rooms; finish with trim/waste.
Tools and touchpoints
- Single-use blades for cuts; dispose after each plant (or each bench at minimum).
- Where re-use is necessary, use validated inactivation (see “Mitigation” below) between plants.
- Benches & trellis: smooth, cleanable surfaces; avoid crack/crevice accumulation points.
Rooms and space
Having the right building layout reduces risk and downtime:
- Quarantine & sampling bays near intake.
- Dedicated sanitation stations: chemical and heat treatment for tools, clearly labeled.
- Propagation & mother rooms with controlled access, handwash/ glove stations at entry, and line-of-travel separation.
- Waste management flow that avoids crossing clean corridors.
If your current shell makes clean zoning impossible, consider moving to an industrial layout designed for controlled-environment agriculture. → Explore compliant warehouse/industrial space for lease or → review buildings for purchase
Mitigation: triage, sanitation that works, and clean-stock rebuilds
Triage and containment
- Immediate isolation of suspected plants/benches; flag irrigation zones and worker access.
- Index (test) surrounding plants and mother lineage.
- Cull confirmed positives; bag plant waste; sanitize contact surfaces.
Sanitation that actually inactivates HLVd
Not all common greenhouse disinfectants work on viroids. Current extension guidance and peer-reviewed work support:
- Bleach (sodium hypochlorite) at 5–10% (or 10–20% household bleach) for blades and hard surfaces with sufficient contact time; rinsing after is recommended for corrosion control. PMCTUMI Genomics
- Dry heat for metal blades: 320 °F (~160 °C) for 10 min between plants is effective; ethanol, Virkon, and hydrogen peroxide are not reliable for HLVd inactivation; autoclaving is also not effective. OSU Extension Service
Practical cadence: Single-use blades are optimal. If re-using, maintain two blade sets—one soaking in fresh bleach while the other is used—then rinse/dry and bake per cycle.
Water & nutrient solution
- Avoid co-rooting infected and clean plants in shared rooting vessels.
- Disinfect or segregate recirculated solutions (if you must recirc) and sanitize reservoirs and lines on a schedule; HLVd can move via roots and shared solutions. MDPI
Clean-stock rebuilds
- Meristem tip culture with thermotherapy can produce viroid-free mother plants in many cultivars (success varies by genotype). Build a clean foundation from verified meristem-derived stock and retire legacy mothers. SpringerLinkPMC
- Seed programs: while low-rate seed transmission can occur, seed lots from clean, indexed parents are a rapid way to refresh genetic libraries while tissue culture pipelines spin up.
- Supplier requirements: demand COA-style HLVd-negative attestations, sampling SOPs, and lot traceability from clone vendors.
Decision matrix and SOPs you can deploy this week
Decision matrix: respond by facility stage
| Situation | Action | Why |
|---|---|---|
| One bench shows early dudding | Isolate bench; RT-qPCR composite sampling (bench ends & symptomatic plants); sanitize tools & surfaces; switch to single-use blades | Limits spread while you confirm; quick containment buys time |
| Positives in mother room | Cull positives; index every mother 2× over 14 days; restrict cut production; begin clean-stock sourcing | Mother infection seeds every room; stop the pipeline |
| Multiple rooms positive | Stand up quarantine bay; suspend inter-room labor movement; escalate sanitation (bleach/heat); audit glove & blade change discipline | Break mechanical transmission chains |
| Chronic reinfection despite sanitation | Review line-of-travel and room adjacencies; assess co-rooting and recirc solution; plan meristem/seed rebuild; consider facility retrofit or relocation | Structural issues require structural fixes; new shell may be cheaper |
SOP: HLVd-Focused Tool Sanitation (Blades & Snips)
Purpose: Prevent mechanical transmission of HLVd during cutting, topping, and pruning.
Scope: All cutting tools in mother, clone, veg, and flower rooms.
PPE: Gloves, eye protection. Use in a ventilated area away from plants.
Materials:
- Freshly mixed 10% household bleach (or 5–10% sodium hypochlorite), labeled with date/time.
- Rinse water (RO preferred) and clean towels.
- Heat unit (dry-heat oven) capable of 320 °F (160 °C).
- Spare tool set to maintain cadence.
Procedure:
- After each plant (minimum: each bench), submerge blades in bleach for ≥1 minute contact.
- Rinse, dry thoroughly to avoid corrosion.
- Bake at 320 °F for 10 minutes.
- Resume work with sanitized tool; rotate sets so one is always sanitizing while the other is in use.
- Replace bleach every 2 hours or sooner if contaminated.
- Log cycle counts per room and keep records with date, operator initials.
Notes: Do not rely on ethanol, Virkon, hydrogen peroxide, or autoclaving for HLVd control. OSU Extension Service
SOP: Mother Indexing & Quarantine Intake
Purpose: Keep mother stock HLVd-free and block entry of infected clones.
Cadence:
- Mothers: RT-qPCR every 2–4 weeks; two negatives 7–14 days apart for release after any quarantine.
- Inbound lots: Quarantine on arrival; pull composite petiole samples per lot; no cuts until negative report.
Records: Maintain lot IDs, sampling dates, lab IDs, and results; retire mother lines after two positives or repeated inconclusive results.
Facility & siting considerations (zoning, buffers, and build-outs)
Biosecurity is easier in the right building:
- Zoning & buffers: Confirm cultivation/manufacturing uses are permitted or conditional in the district, and document buffer distances and measurement method with the AHJ (commonly 600–1,000 ft to sensitive uses).
- Flow-friendly layout: Seek shells with clear separations for quarantine/sampling, mother & propagation, and production rooms.
- Utilities & space: Dedicated areas for sanitation (chemical and heat), handwash/glove stations at entries, and smooth, cleanable surfaces.
- Drainage & recirc plumbing: Design out co-rooting points and risky recirculation; if recirc is necessary, plan for solution sanitation and reservoir isolation.
- Contingency (acquire vs. rehab): If your current shell can’t support clean flow, consider acquiring a compliant operation and retiring infected stock. → See cultivation/production businesses for sale
- Speed to open: Where new build-out delays threaten revenue, lease a ready industrial space optimized for CEA while your clean-stock pipeline spins up. → Browse industrial grow spaces for lease and → industrial for sale
Next steps and curated inventory for faster recovery
- Run a baseline index this week: mothers, inbound lots, and one symptomatic bench per room.
- Stand up sanitation that works: switch to single-use blades where possible; bleach + heat cadence elsewhere.
- Publish the two SOPs above to your team; track compliance with simple checkboxes and weekly spot audits.
- Begin clean-stock planning: tissue culture/meristem pipeline and vendor requirements; retire legacy mothers methodically.
- Evaluate the building: if flow is fighting you, move to a shell that supports clean zoning—or acquire a performing asset and convert it.
Ready to act?
→ Evaluate operating cultivation businesses you can acquire
→ Browse compliant industrial shells for lease
→ Review industrial buildings for purchase
Disclaimer
This article is for educational purposes only and does not constitute legal, engineering, financial, or tax advice. Always consult qualified professionals and your local Authority Having Jurisdiction before making decisions.
Please visit:
Our Sponsor